The collisional integral of Boltzmann equation for hard spheres,
Eq. (2.76), contains a term
![]() which multiplies the probabilities of
particles entering or coming out from a collision. In general the
collisional integral must contain the differential collision rate
which multiplies the probabilities of
particles entering or coming out from a collision. In general the
collisional integral must contain the differential collision rate
![]() for particle coming at a certain relative velocity (in
modulus
for particle coming at a certain relative velocity (in
modulus ![]() and direction
and direction
![]() , or equivalently
scattering angle
, or equivalently
scattering angle ![]() centered in the solid angle
centered in the solid angle ![]() ), which
may be expressed in terms of the scattering cross section
), which
may be expressed in terms of the scattering cross section ![]() (see for
example Eq. (2.20)):
(see for
example Eq. (2.20)):
 |
(2.84) |
We discussed in paragraph 2.1.2 the fact that the
scattering cross section depends strongly on the kind of interaction
between the molecules of the gas. For power law repulsive interaction
potential
![]() , the scattering angle
, the scattering angle ![]() depends
on the relative energy
depends
on the relative energy ![]() and on the impact parameter
and on the impact parameter ![]() only
through the combination
only
through the combination
![]() (see for
example [59]). This means that there exists a function
(see for
example [59]). This means that there exists a function
![]() such that:
such that:
| (2.85) |
and this means that from relation (2.22) one obtains:
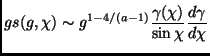 |
(2.86) |
which holds in ![]() . The extension to generic dimension of the last equation is:
. The extension to generic dimension of the last equation is:
 |
(2.87) |
Therefore when
![]() (i.e.
(i.e. ![]() for
for ![]() and
and ![]() for
for
![]() ) the collision rate
) the collision rate
![]() does not depend upon
does not depend upon ![]() .
This property defines the so-called Maxwell molecules [81]. Interaction
with
.
This property defines the so-called Maxwell molecules [81]. Interaction
with
![]() are called soft interactions (e.g. the electrostatic
or gravitational interaction). Interactions with
are called soft interactions (e.g. the electrostatic
or gravitational interaction). Interactions with
![]() are
called hard interactions. Hard spheres (
are
called hard interactions. Hard spheres (
![]() ) belongs to
this set of interactions, with
) belongs to
this set of interactions, with
![]() . It has been also
studied the Very Hard Particles model, which is characterized by
. It has been also
studied the Very Hard Particles model, which is characterized by
![]() , which is not attainable with an inverse power
potential, as it requires an interaction harder than the hard sphere
interaction.
, which is not attainable with an inverse power
potential, as it requires an interaction harder than the hard sphere
interaction.
The obvious advantage of Maxwell molecules is that the Boltzmann
equation greatly simplifies, as ![]() does not appear in the collision
integral. A further simplification of the Boltzmann equation came from
Krook and Wu [129], who studied the Boltzmann equation of
Maxwell molecules with an isotropic scattering cross-section, i.e
does not appear in the collision
integral. A further simplification of the Boltzmann equation came from
Krook and Wu [129], who studied the Boltzmann equation of
Maxwell molecules with an isotropic scattering cross-section, i.e
![]() , often called Krook and Wu model. A very large
literature exists for linear and non-linear model-Boltzmann equations
(for a review see [81]). The importance of the Maxwell
molecules model is the possibility of obtaining solutions for it: the
general method (extended to other model-Boltzmann equations) is to
obtain an expansion in orthogonal polynomial where the expansion
coefficients are polynomial moments of the solution distribution
function. For Maxwell molecules the moments satisfy a recursive system
of differential equations that can be solved sequentially. Given an
initial distribution, one can solve the problem if the series
expansion converges. Bobylev [32] has shown that if one
searches for similarity solutions (i.e. solutions with scaling
form
, often called Krook and Wu model. A very large
literature exists for linear and non-linear model-Boltzmann equations
(for a review see [81]). The importance of the Maxwell
molecules model is the possibility of obtaining solutions for it: the
general method (extended to other model-Boltzmann equations) is to
obtain an expansion in orthogonal polynomial where the expansion
coefficients are polynomial moments of the solution distribution
function. For Maxwell molecules the moments satisfy a recursive system
of differential equations that can be solved sequentially. Given an
initial distribution, one can solve the problem if the series
expansion converges. Bobylev [32] has shown that if one
searches for similarity solutions (i.e. solutions with scaling
form
![]() ), then the solution can be found solving a recursive
system of algebraic equation.
), then the solution can be found solving a recursive
system of algebraic equation.
The Maxwell molecules model has been subject of study also in the framework of the kinetic theory of granular gases. In section 5.3 we discuss this issue.