The concept of mean free path was introduced in 1858 by Rudolf
Clausius [65] and paved the road to the development of
the kinetic theory of gas. For the sake of simplicity (and coherently
with the rest of this work, as well as with the literature on granular
gases) we consider a single species gas composed of hard spheres, all
having the same diameter ![]() and mass
and mass ![]() (see [62]
or [98]).
(see [62]
or [98]).
The mean free time is the average time between two successive
collisions of a single particle. We define ![]() the probability
that a given particle suffers a collision between time
the probability
that a given particle suffers a collision between time ![]() and
and ![]() (
(![]() is called collision frequency) and assume that
is called collision frequency) and assume that ![]() is
independent of the past collisional history of the particle. The
probability
is
independent of the past collisional history of the particle. The
probability
![]() of having a free time between two successive
collisions larger than
of having a free time between two successive
collisions larger than ![]() and shorter than
and shorter than ![]() is equal to the
product of the probability that no collision occurs in the time
interval
is equal to the
product of the probability that no collision occurs in the time
interval ![]() and the probability that a collision occurs in the
interval
and the probability that a collision occurs in the
interval ![]() :
:
| (2.17) |
where
![]() is the survival probability, that is the
probability that no collisions happen between 0 and
is the survival probability, that is the
probability that no collisions happen between 0 and ![]() , and can be
calculated observing that
, and can be
calculated observing that
![]() so that
so that
![]() , i.e.
, i.e.
![]() .
.
Finally one can calculate the average of the free time using the
probability density
![]() :
:
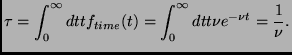 |
(2.18) |
With the same sort of calculations an expression for the mean free
path, that is the average distance traveled by a particle between two
successive collisions, can be calculated. One again assumes that there
is a well defined quantity (independent of the collisional history of
the particle)
![]() which is the probability of a
collision during the travel between distances
which is the probability of a
collision during the travel between distances
![]() and
and
![]() . The survival probability in terms of space traveled is
. The survival probability in terms of space traveled is
![]() and the probability
density of having a free distance
and the probability
density of having a free distance
![]() is
is
![]() so that the mean free path is given by:
so that the mean free path is given by:
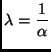 |
(2.19) |
The other important statistical quantity in the study of binary
collisions is the so-called differential scattering cross
section ![]() which is defined in this way: in a unit time a particle
suffers a number of collisions which can be seen as the incidence of
fluxes of particles coming with different approaching velocities
which is defined in this way: in a unit time a particle
suffers a number of collisions which can be seen as the incidence of
fluxes of particles coming with different approaching velocities
![]() and scattered to new different departure velocities
and scattered to new different departure velocities
![]() . Given a certain approaching velocity
. Given a certain approaching velocity
![]() the incident particles arrive with slightly different impact
parameters (due to the extension of the particles), and therefore are
scattered in a solid angle
the incident particles arrive with slightly different impact
parameters (due to the extension of the particles), and therefore are
scattered in a solid angle ![]() . If
. If ![]() denotes the intensity
of the beam of particles that come with an average approaching speed
denotes the intensity
of the beam of particles that come with an average approaching speed
![]() , which is the number of particles intersecting in unit
time a unit area perpendicular to the beam (
, which is the number of particles intersecting in unit
time a unit area perpendicular to the beam (
![]() with
with ![]() the
number density of the particles), then the rate of scattering
the
number density of the particles), then the rate of scattering ![]() into the
small solid angle element
into the
small solid angle element ![]() is given by
is given by
where ![]() is a factor of proportionality with the dimensions of an
area (in
is a factor of proportionality with the dimensions of an
area (in ![]() ) which is called differential cross section and depends
on the relative velocity vectors before and after the collisions. The
total rate of particles scattered in all directions,
) which is called differential cross section and depends
on the relative velocity vectors before and after the collisions. The
total rate of particles scattered in all directions, ![]() is the
integral of the last equation:
is the
integral of the last equation:
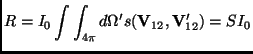 |
(2.21) |
and defines the total scattering cross section ![]() .
.
In the case of a spherically symmetric central field of force the
differential cross section is a function only of the modulus of the
initial relative velocity ![]() , the angle of deflection
, the angle of deflection ![]() , and
the impact parameter
, and
the impact parameter ![]() which in turn, once fixed the potential
which in turn, once fixed the potential
![]() , is a function only of
, is a function only of ![]() and
and ![]() , that is
, that is
![]() . In particular it can be easily demonstrated that
. In particular it can be easily demonstrated that
The case of inverse power interaction potential is of interest also
for the study of cross sections: a very famous result in this
framework is the Rutherford formula that concerns the differential
cross section for the case ![]() (scattering of an electron by an
atomic nucleus):
(scattering of an electron by an
atomic nucleus):
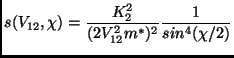 |
(2.23) |
where
![]() (for the electron charge
(for the electron charge ![]() and the
vacuum permittivity
and the
vacuum permittivity
![]() ).
).
In addition to the differential and total scattering cross sections, in non-equilibrium transport theory several other cross sections are defined:
where ![]() is a positive integer (n=1,2,....). For instance, the
transfer of the parallel component of the particle momentum is
proportional to
is a positive integer (n=1,2,....). For instance, the
transfer of the parallel component of the particle momentum is
proportional to
![]() (see Eq. (2.10)) and
therefore
(see Eq. (2.10)) and
therefore ![]() is related to the transport of momentum and plays an
important role in the study of diffusion. Moreover, viscosity and heat
conductivity depend on
is related to the transport of momentum and plays an
important role in the study of diffusion. Moreover, viscosity and heat
conductivity depend on ![]() .
.
To conclude this paragraph we recall that the collision frequency defined at the beginning is strictly tied to the total scattering cross section by the relation
| (2.25) |
where ![]() is the average density of the gas and
is the average density of the gas and
![]() is
an average of the relative velocities. Generally speaking (in the
framework of a non-equilibrium discussion)
is
an average of the relative velocities. Generally speaking (in the
framework of a non-equilibrium discussion) ![]() and
and
![]() are averages taken in space-time regions in which equilibrium can be
assumed. Assuming that in this region the distribution of velocities
of the particle is the Maxwell-Boltzmann distribution:
are averages taken in space-time regions in which equilibrium can be
assumed. Assuming that in this region the distribution of velocities
of the particle is the Maxwell-Boltzmann distribution:
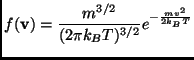 |
(2.26) |
the collision frequency can be calculated obtaining the formula:
| (2.27) |
where
![]() is the average of the modulus of the velocities and, in this case, is given by:
is the average of the modulus of the velocities and, in this case, is given by:
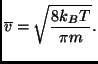 |
(2.28) |
In the same way the mean free path is given by