In the kinetic theory the condition of equilibrium is equivalent to
the absence of macroscopic flows, i.e. absence of transport. The
transport of the molecular quantity ![]() along a particular direction
along a particular direction
![]() is characterized by its net flux
is characterized by its net flux
![]() which is defined as the net fraction of
which is defined as the net fraction of ![]() crossing
in the unit time a unit surface normal to the direction
crossing
in the unit time a unit surface normal to the direction ![]() in
the point
in
the point
![]() of the space. If the quantity
of the space. If the quantity ![]() is always
transported by molecular motion or transferred from a particle to
another via collision interactions that conserve the sum of
is always
transported by molecular motion or transferred from a particle to
another via collision interactions that conserve the sum of ![]() (i.e.
(i.e. ![]() is said to be a collisional invariant), then the
variation in time of the coarse grained field
is said to be a collisional invariant), then the
variation in time of the coarse grained field
![]() (which
is an average of
(which
is an average of ![]() taken on particles in a well suited region of
space-time centered in
taken on particles in a well suited region of
space-time centered in
![]() ) is simply expressed by a
continuity formula:
) is simply expressed by a
continuity formula:
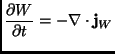 |
(2.35) |
In elastic gases the relevant conserved molecular quantities are: ![]() ,
,
![]() and
and ![]() , that is mass, momentum and energy.
, that is mass, momentum and energy.
Empirical observations show that, in situations not too far from equilibrium almost all the transport fluxes are proportional to the spatial gradient of the transported quantity:
| (2.36) |
where ![]() is called the transport coefficient for the quantity
is called the transport coefficient for the quantity
![]() . The most important transport coefficients are: the diffusion
coefficient
. The most important transport coefficients are: the diffusion
coefficient ![]() (transport of mass or self diffusion), the viscosity
(transport of mass or self diffusion), the viscosity ![]() (transport
of transversal momentum, e.g. transport of
(transport
of transversal momentum, e.g. transport of ![]() in the direction
in the direction ![]() or
or ![]() ) and the heat conductivity
) and the heat conductivity ![]() (transport of heat, that is
internal energy). The flux of longitudinal momentum (e.g.
(transport of heat, that is
internal energy). The flux of longitudinal momentum (e.g. ![]() in the
direction
in the
direction ![]() ) is somewhat different as it is dominated by an order
zero (in the spatial gradients) contribution which is given by the
hydrostatic pressure
) is somewhat different as it is dominated by an order
zero (in the spatial gradients) contribution which is given by the
hydrostatic pressure ![]() (in the Boltzmann-Grad limit
(in the Boltzmann-Grad limit ![]() ).
).
Starting from the basic concepts of mean free time and path, it is possible to derive heuristic expressions for the transport coefficients [62]. Such calculations, that take the name of mean free path method are meaningful in the limit of small Knudsen numbers:
where ![]() is the mean free path and
is the mean free path and ![]() is the characteristic
linear size of the problem (related to boundary conditions, e.g. in a
shear experiment
is the characteristic
linear size of the problem (related to boundary conditions, e.g. in a
shear experiment
![]() where
where
![]() is the
thermal velocity of the fluid and
is the
thermal velocity of the fluid and
![]() is the
shear rate). This condition corresponds to the necessity that the
variation of all macroscopic quantities is small within a mean free
path.
is the
shear rate). This condition corresponds to the necessity that the
variation of all macroscopic quantities is small within a mean free
path.
With this assumption, very rough calculations carry to good estimates of the main transport coefficients:
In the last formulas the rightmost sides are calculated using the
expressions for the mean free path and for the average velocity
modulus obtained with the assumption of Maxwell-Boltzmann equilibrium.
In the above equations one sees that the viscosity and the heat
conductivity do not depend on the density, but only on the temperature
of the gas. Apparently one expects that the viscosity depends upon the
density (more particles carry more momentum) but in these approximated
calculations the fluxes are assumed to be carried only by molecular
motion (i.e. collisional transfer is negligible): for the viscosity
for example this means the appearance of the product ![]() which does not
depend on
which does not
depend on ![]() (as
(as ![]() is inversely proportional to
is inversely proportional to ![]() ).
).