| (2.101) |
We assume that a phase space distribution function can be defined:
| (2.101) |
where
![]() is the number of particles found at
time
is the number of particles found at
time ![]() near the point
near the point
![]() of the phase
space.
of the phase
space. ![]() is assumed to be the solution of the Boltzmann Equation
(2.74).
is assumed to be the solution of the Boltzmann Equation
(2.74).
The particle number density is defined as
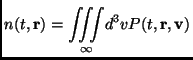 |
(2.102) |
The average molecular velocity is defined as
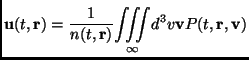 |
(2.103) |
and this allows to introduce the random velocity vector
| (2.104) |
which depends on time and position (while
![]() is independent
of
is independent
of ![]() and
and
![]() ) and has zero average:
) and has zero average:
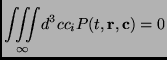 |
(2.105) |
The average fluxes of the molecular quantity
![]() can be
expressed as velocity moments of the phase space distribution
function:
can be
expressed as velocity moments of the phase space distribution
function:
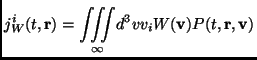 |
(2.106) |
When ![]() one has the mass flux:
one has the mass flux:
| (2.107) |
When ![]() one has the momentum flux:
one has the momentum flux:
| (2.108) |
which is a
![]() symmetric matrix. In the last form two
contributions can be recognized, that is the flux due to the bulk
(organized) motion and the flux
resulting from the random (thermal) motion of the gas particles. This
second term is usually called the pressure tensor
symmetric matrix. In the last form two
contributions can be recognized, that is the flux due to the bulk
(organized) motion and the flux
resulting from the random (thermal) motion of the gas particles. This
second term is usually called the pressure tensor
![]() . One can define, from this discussion, two
quantities that are the scalar pressure
. One can define, from this discussion, two
quantities that are the scalar pressure ![]() and the vector temperature
and the vector temperature ![]() :
:
and in the isotropic case ![]() so that
so that ![]() . It can be also
defined the stress tensor
. It can be also
defined the stress tensor
![]() as:
as:
which expresses the deviation of the pressure tensor from the
equilibrium Maxwellian case (for which
![]() ).
).
Finally, the flux of the quantity ![]() is given by:
is given by:
where
![]() is the
generalized heat flow tensor and describes the transport of random
energy
is the
generalized heat flow tensor and describes the transport of random
energy ![]() due to thermal motion
due to thermal motion ![]() of the molecules (for all
the permutations of
of the molecules (for all
the permutations of ![]() ).
).
In equation (2.118) three contributions can be recognized: the first term describes the bulk transport of the bulk flux of momentum; the second, third and fourth terms describe the a combination of bulk and random momentum fluxes; the last term is the transport of random energy component due to the random motion itself. Often a ``classical'' heat flow vector is introduced, more intuitive than the generalized heat flow tensor:
It is also of use to give the definition of the fourth velocity moment: